MATTER?
----Anything that has mass and takes up space.
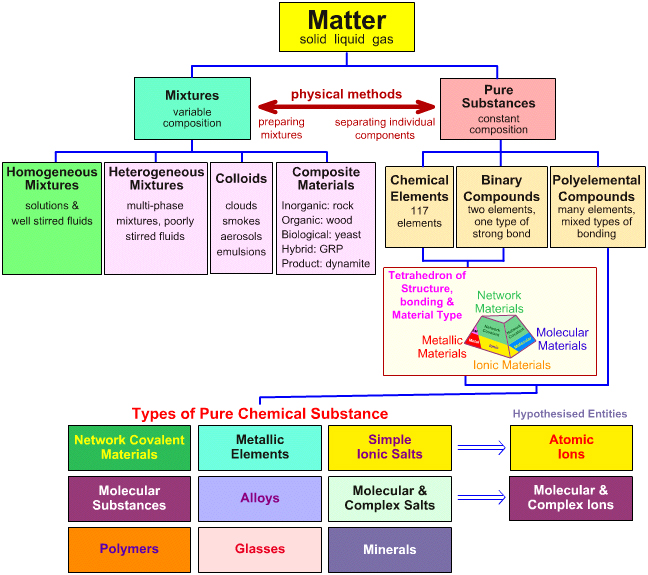
Matter
includes
1.Pure Substances
---one set of properties
---one kind of particle
---simplest form(cannot be decomposed)
---made of atoms 1.metal 2.nonmetal 3.metalloid
(2)compounds----made of elements
---smallest particle is a molecule(Ionic & Covalent)
2.Mixtures
---more than one set of properties and substances
---uniform throughout
---appears to have only one component
Ex: Solutions & colloid
---not uniform---appears to have more than one component
Ex: Salad Dressing (Suspensior & Mechanical Mixture)
Water+oil
Physical Change vs. Chemical Change
Physical Change---no new substance is formed
---chemical composition doesn't change
---reversible
Chemical Change
---new substances are formed
---irreversible
A few more properties for matter
---it is neither created nor destroyed(only changed form from one form to another)
Three States
(1)Solid
(2)Liquid
---takes the shape of the container and experiences slight changes in volume when heated
(3)Gases
---takes the shape of the container and experiences drastic change in volume when heated

没有评论:
发表评论