The empirical formula of an organic compound(any substance that contains carbon) can be found by
-Burning the compound(reacting it with O2).
-Collecting ang weighing the products.
-From the mass of the product,the moles of each element in theoriginal organic compound can be calculated.
2011年12月5日星期一
2011年12月1日星期四
%Composition to Empirical Formula
From the % composition, the ratio of moles can be dertermined . This will lead to the empirical formula.
Assume 100.0g of material.
Convert all % is to grams.
Convert all grams to moles.
Divide all moles by the smallest.
Value,this will give an X:1 ratio to determine the empirical formula.
Assume 100.0g of material.
Convert all % is to grams.
Convert all grams to moles.
Divide all moles by the smallest.
Value,this will give an X:1 ratio to determine the empirical formula.
2011年11月30日星期三
Percent Composition
- The % by mass of the elements in a compound is called the percent composition
How to find the percent composition: 1. Calculate molar mass
2. Calculate each element percentage of that total
Give percentage to 1decimal place
-Percent composition tells what part of a compound each component makes up
Ex1. What is the % composition of CO2?
1 mole C= 1×12.0= 12.0g
2mole O= 2 ×16.0= 32.0g
44.0g
%C= (mass of carbon)/(mass of CO2)=(12.0×100%)/44.0g=27.3%
%O= (mass of oxygen)/(mass of CO2)= (32.0×100%)/44.0g=72.7%
%C= (mass of carbon)/(mass of CO2)=(12.0×100%)/44.0g=27.3%
%O= (mass of oxygen)/(mass of CO2)= (32.0×100%)/44.0g=72.7%
Check to see if the result is close to 100%:27.3%+72.7%= 100%
Because of rounding answer may also be shown as 99.9 and 100.1 which are both OK
Ex2. What is the % composition of the entire element in glucose: C6H12O6
C = 72.0g H=12.0g O= 96.0g Total Mass=180.0g
%C= (72.0g×100%)/180.0g= 40.0%
%H= (12.0g×100%)/180.0g= 6.7%
%O= (96.0g×100%)/180.0g= 53.3%
40.0+6.7+53.3=100%
%H= (12.0g×100%)/180.0g= 6.7%
%O= (96.0g×100%)/180.0g= 53.3%
40.0+6.7+53.3=100%
To find the percent composition of a group of atoms (Al2(SO4)3), the total of the mass of the group atom is used for the percentage
Ex3. Find the % SO4 in Al2(SO4)4
2Al× 27.0g= 54g
3S × 32.1g= 96.3g 96.3(Sulphur)+192.0(Oxygen)=288.3g
12O× 16.0g= 192.0g
342.3g
%SO4= 288.3g/342.3×100%= 84.2%SO4
From the percent composition, ratio of moles can be determined and this will result in an empirical formula
From the percent composition, ratio of moles can be determined and this will result in an empirical formula
Step 1: Assume 100.0g of material
Step 2: Convert all %s to grams Step 3: Convert grams to moles
Step 4: divide all moles by the smallest; this will give you a ratio to determine the empirical formula
Ex4. Sample of compound: 45.8% Sulphur and 54.2% Flouride. Give the empirical formula
Step 1: Assume the 100.0g of material
Step 2: Therefore: 45.8g S and 54.2g F
Step 3: 45.8/32.1g= 1.4268molS 54.2/19.0g= 2.8526molF
Step 4: 2.8526/1.4268= 1.99993/(1moleS)= SF2
Therefore the empirical formula for 45.8% Sulphur and 54.2% Flouride is SF2
How to calculate percent composition1: V=xbEeyT8nK84
How to calculate percent composition2: V=_H009sTvYE0
Therefore the empirical formula for 45.8% Sulphur and 54.2% Flouride is SF2
How to calculate percent composition1: V=xbEeyT8nK84
How to calculate percent composition2: V=_H009sTvYE0
2011年11月22日星期二
Graph-Lab
Use 'Microsoft Office Excel 2007' to draw the graphs!!!!
There are three variables: Mass, Temperature, Density, Volume
Draw 3 kinds of graphs: Mass VS Volume
Temp VS Density
Volume VS Temp
First, list the statistics:
Example:
Draw the graphs by the graphing tools in Excel:

Lets see a teaching video about how to use Excel Graphing tool:
There are three variables: Mass, Temperature, Density, Volume
Draw 3 kinds of graphs: Mass VS Volume
Temp VS Density
Volume VS Temp
First, list the statistics:
Example:
| TEMP | VOLUME |
| 273 | 152 |
| 320 | 175 |
| 365 | 203 |
| 412 | 226 |
| 455 | 249 |
| 521 | 273 |
| 546 | 302 |
| 580 | 323 |
| 632 | 351 |
Draw the graphs by the graphing tools in Excel:

Solve the slope:
| Slope | 0.554318 |
Lets see a teaching video about how to use Excel Graphing tool:
Significant Figures
Significant Figures?-Measure or meaningful digits.
-More preise mensthere are more significant digits.

1.Significant Digits-The last digit in a measurement is uncertain as it could be one higher or one digit lower very eaily.
Eg. 2.78g on a balance : the 2&7 are CERTAIN number and 8 is UNCERTAIN
-The significant digits in the measurement includes all of the certain digits plus only the first uncertain digits.
Eg. the 2.78g has "3" significant digits
2.Significant Figures:Details-Leading zero(s) aren't counted
Eg.0.01 has ONE siginificant digit
-Trailing zero(s) after the decimal point are counted
Eg.1.000 000 has SEVEN significant digits.
-Trailing zero(s) without a decimal point are NOT counted
Eg.1900 has Two significant digit.

3.Exact Numbers-Some quantities are defined as exactly a certain amount and rounding is required.
Eg. A "pair" of shoes=2 shoes
4.Rounding Rules-We round answers to the appropriate number of digits using rules similar to math with one set of exception.
-Look at the digits after the position of rounding.
1.Digit >5 , round up
Eg.64.36=64.4
2.Digit <5, round down
64.33=64.3
3.①Digit=5, more numbers (non-zero) after 5, round up
Eg. 64.35123=64.4
②Digit=5, ends at the 5, round to make the last digit EVEN(0.2.4.6.8)
Eg. 64.35=64.4
64.55=64.6
5.Math Rules 1. “+”&“--"-When adding & subtracting, round to the fewest number of decial places
Eg 12.333
+ 20.1
-----------------
32.433
Round to 1 decimal place, the answer=32.4
Eg 12500
+ 6000
---------------
18500
Round to the thousands place (the first uncertain digit)=18000
6.Math Rules2 "× " & "÷"-When multiplying or dividing, round to the fewest number significant
Eg. 12.01 4 sig figures
× 1.5 2 sig figures
------------------------
6.005
+ 12.01
---------------------------
18.015
Round to 2 sig figures (2<4) or the 1st uncertain digit.
Answer=18.02
Eg. Long Division
12.54÷1.3=9.64
Round to 2 sig figures
Answer=9.6
-More preise mensthere are more significant digits.

1.Significant Digits-The last digit in a measurement is uncertain as it could be one higher or one digit lower very eaily.
Eg. 2.78g on a balance : the 2&7 are CERTAIN number and 8 is UNCERTAIN
-The significant digits in the measurement includes all of the certain digits plus only the first uncertain digits.
Eg. the 2.78g has "3" significant digits
2.Significant Figures:Details-Leading zero(s) aren't counted
Eg.0.01 has ONE siginificant digit
-Trailing zero(s) after the decimal point are counted
Eg.1.000 000 has SEVEN significant digits.
-Trailing zero(s) without a decimal point are NOT counted
Eg.1900 has Two significant digit.

3.Exact Numbers-Some quantities are defined as exactly a certain amount and rounding is required.
Eg. A "pair" of shoes=2 shoes
4.Rounding Rules-We round answers to the appropriate number of digits using rules similar to math with one set of exception.
-Look at the digits after the position of rounding.
1.Digit >5 , round up
Eg.64.36=64.4
2.Digit <5, round down
64.33=64.3
3.①Digit=5, more numbers (non-zero) after 5, round up
Eg. 64.35123=64.4
②Digit=5, ends at the 5, round to make the last digit EVEN(0.2.4.6.8)
Eg. 64.35=64.4
64.55=64.6
5.Math Rules 1. “+”&“--"-When adding & subtracting, round to the fewest number of decial places
Eg 12.333
+ 20.1
-----------------
32.433
Round to 1 decimal place, the answer=32.4
Eg 12500
+ 6000
---------------
18500
Round to the thousands place (the first uncertain digit)=18000
6.Math Rules2 "× " & "÷"-When multiplying or dividing, round to the fewest number significant
Eg. 12.01 4 sig figures
× 1.5 2 sig figures
------------------------
6.005
+ 12.01
---------------------------
18.015
Round to 2 sig figures (2<4) or the 1st uncertain digit.
Answer=18.02
Eg. Long Division
12.54÷1.3=9.64
Round to 2 sig figures
Answer=9.6
Aluminum Foil Thickness Lab

Objective: To calculate the thickness of a sheet of aluminum foil and express the answer in terms of proper scientific notation and significant figures
Supplies: 3 rectangular pieces of aluminum foil (minimum 15cm*15cm); metric ruler and centigram balance.
Experimental results:
Table 1
sheet Length(cm) Wideth(cm) Mass(g)
1 16.8 15.4 1.02
2 15.8 15.0 0.95
3 17.2 17.0 1.16
Formulars:
Volume of a rectangular solid: V=L*W*H
Density: D=M/V
H(average)=(H1+H2+H2)/3
Example:
Sheet1: 2.70g/cm^3=1.02g/(16.8cm*15.4cm*H) H1=1.46*10^-3cm
2011年11月9日星期三
Density
What is Density?
- a physical property of matter that describes the ratio of mass to volume
D=M/V
Possible units: g/ml, g/L, g/cm³, kg/L
Important: 1cm³=1ml
Density of water=1.0g/ml or 1000g/L
Density object > density liquid objects sinks
Density object < density liquid object floats
Ex. Iron bar has mass of 1.2kg and a volume of 1.25L. What is it’s density?
D=M/V=1.2kg/1.25L=0.96Kg/L
Lets watch a video about Density demonstration:
- a physical property of matter that describes the ratio of mass to volume
D=M/V
Possible units: g/ml, g/L, g/cm³, kg/L
Important: 1cm³=1ml
Density of water=1.0g/ml or 1000g/L
Density object > density liquid objects sinks
Density object < density liquid object floats
Ex. Iron bar has mass of 1.2kg and a volume of 1.25L. What is it’s density?
D=M/V=1.2kg/1.25L=0.96Kg/L
Lets watch a video about Density demonstration:
Measurements
-no measurement is exact, but there is an estimate of the measurement
-When measuring, at least measure 3 times and calculate the average from the 3 or more
-absolute uncertainty is the largest difference between the average and the lowest and highest reasonable measurement
-biggest difference and smallest difference is usually shown with a positive sign on top and a negative sign on the bottom (±)
Ex. 35.3 ± 0.2, either 35.1 or 35.5
Ex2. 29.95 ± 0.002, either 29.97 or 29.93
Measuring Device Smallest division Measurement nearest Uncertainty
Ruler 0.1 0.01cm 0.01cm
Thermometer 1°C 0.1° 0.1°
100ml graduated cylinder 1ml 0.1ml 0.1ml
400ml beaker 50ml 5ml 5ml
Relative uncertainty=absolute uncertainty/estimated measurement
-When measuring, at least measure 3 times and calculate the average from the 3 or more
-absolute uncertainty is the largest difference between the average and the lowest and highest reasonable measurement
-biggest difference and smallest difference is usually shown with a positive sign on top and a negative sign on the bottom (±)
Ex. 35.3 ± 0.2, either 35.1 or 35.5
Ex2. 29.95 ± 0.002, either 29.97 or 29.93
Measuring Device Smallest division Measurement nearest Uncertainty
Ruler 0.1 0.01cm 0.01cm
Thermometer 1°C 0.1° 0.1°
100ml graduated cylinder 1ml 0.1ml 0.1ml
400ml beaker 50ml 5ml 5ml
Relative uncertainty=absolute uncertainty/estimated measurement
2011年10月19日星期三
Lab Safety
Lab Safety

1. Report all accidents regardless of how minor to your teacher.
2. Work in the lab only when the teacher is present or when you have permission to do so.
3. Never indulge in horseplay or behavior that could lead to injury of others.
4. Before beginning work in lab, clean the lab bench top and your glassware.
5. Use goggles and lab aprons when instructed to do so.
6. Due to the dangers of broken glass and corrosive liquid spills in the lab, open sandals or bare feet are not permitted in the lab.
7. Learn the location and proper usage of the eyewash fountain, fire extinguisher, safety shower, fire alarm box, office intercom button, evacuation routes, clean-up brush and dust pan, glass/chemical disposal can.
8. For minor skin burns, immediately plunge the burned area into cold water and notify the teacher.
9. If you get any chemical in your eye, immediately wash the eye with the eye-wash fountain and notify the teacher.
10. Never look directly into a test tube. View the contents from the side.
11. Never smell a material in a test tube or flask directly. Instead, with your hand, "fan" some of the fumes to your nose carefully.
12. Immediately notify the teacher of any chemical spill and clean up the spill as directed.
13. Never take chemical stock bottles to the lab benches.
14. Use equipment only as directed:
- a. never place chemicals directly on the pan balances.
b. use glycerin when inserting glass tubing into rubber stoppers.
c. be cautious of glassware that has been heated.
d. add boiling chips to liquid that is to be heated before heating.
e. point test tubes that are being heated away from you and others.
16. Food, drink and gum are prohibited in lab.
17. Never add water to concentrated acid solutions. The heat generated may cause spattering. Instead, as you stir, add the acid slowly to water.
18. Read the label on chemical bottles at least twice before using the chemical. Many chemicals have names that are easily confused.
19. Return all lab materials and equipment to their proper places after use.
20. Upon completion of work, wash and dry all equipment, your lab bench and your clean-up area.
Lab 3B
Separation of a Mixture by Paper ChromatographyChromatography is one technique used by chemists to separate mixture of chemical compounds in order to identify or iso;ate their components on chromatography, mixture are separated according to the different solubilities of the components in liquids, or their adsorption on solids.
The method of identifying components is to calculate the Rf value of each.
Rf=d1/d2 where d1=distance traveled by solute
d2=distance traveled by solvent
the lab report:
Objective: 1. To assemble and operate a paper chromatography apparatus
2. To study the meaning and significance of Rf values
3. To test various food colourings and to calculate their Rf values
4. To compare measured Rf values with standard Rf values
5. To separate mixtures of food colourings into their components
6. To identify the components of mixture by means of their Rf values
Material and Equipment:Refer to page 33 in Health Lab Text, Lab 3B
Procedure:
Refer to page 33 in Health Lab Text, Lab 3B
Data and Observation:Attached to the back of the lab report.(on the paper....)
Analysis of Results:1. A, We tested the yellow colouring. It contains Yellow #6
B. We didn't get any.
2. Green food colouring contains about 1/3 of yellow colouring(Yellow #6) and 2/3 of blue colouring(Blue #2).
3. The unknown mixture is composed of 1/2 of red colouring(Red #4), 1/3 of blue colouring(Blue #2) and 1/6 of Yellow colouring(Yellow #6)
4. The ink mark might be blurred and move up.
5. The green colouring can be decomposed into blue and yellow, but red, yellow and blue colourings cannot be decomposed.
Following-Up Questions1. The Rf of those colourings are 0.78 and 0.38. They are likely to be Red#3 and Yellow #6.
2. d1= 12*1.0 = 12 d2 = 12*0.41 = 4.92 Δd = 12-4.92 = 7.08cm
3. There is no way that a solute move above the solvent. It is impossible.
ConclusionFrom this lab, we got to know the way we separate a mixture by Paper Chromatography. The Rf value means the ratio of distances raveled by solute and solvent. Different substances usually have different Rf values. We can use this property to separate different substances. And we can also identify the substances by checking the table of Rf values.
The method of identifying components is to calculate the Rf value of each.
Rf=d1/d2 where d1=distance traveled by solute
d2=distance traveled by solvent
the lab report:
Objective: 1. To assemble and operate a paper chromatography apparatus
2. To study the meaning and significance of Rf values
3. To test various food colourings and to calculate their Rf values
4. To compare measured Rf values with standard Rf values
5. To separate mixtures of food colourings into their components
6. To identify the components of mixture by means of their Rf values
Material and Equipment:Refer to page 33 in Health Lab Text, Lab 3B
Procedure:
Refer to page 33 in Health Lab Text, Lab 3B
Data and Observation:Attached to the back of the lab report.(on the paper....)
Analysis of Results:1. A, We tested the yellow colouring. It contains Yellow #6
B. We didn't get any.
2. Green food colouring contains about 1/3 of yellow colouring(Yellow #6) and 2/3 of blue colouring(Blue #2).
3. The unknown mixture is composed of 1/2 of red colouring(Red #4), 1/3 of blue colouring(Blue #2) and 1/6 of Yellow colouring(Yellow #6)
4. The ink mark might be blurred and move up.
5. The green colouring can be decomposed into blue and yellow, but red, yellow and blue colourings cannot be decomposed.
Following-Up Questions1. The Rf of those colourings are 0.78 and 0.38. They are likely to be Red#3 and Yellow #6.
2. d1= 12*1.0 = 12 d2 = 12*0.41 = 4.92 Δd = 12-4.92 = 7.08cm
3. There is no way that a solute move above the solvent. It is impossible.
ConclusionFrom this lab, we got to know the way we separate a mixture by Paper Chromatography. The Rf value means the ratio of distances raveled by solute and solvent. Different substances usually have different Rf values. We can use this property to separate different substances. And we can also identify the substances by checking the table of Rf values.
separation
Separation:-Basis for separation: Different components and properties-components in a mixture retain their identities
-the more similar the properties are, the more difficult it is to separate them.
-Strategy: use any possible way to discriminate between components with different properties.
Hand Separation & Evaporation:-Hand separation (Solid+Solid)
-Use a magnet or sieve
-Evaporation(Solid dissolved in liquids) boil away the liquid and the solids remain
Filtration (Solids NOT dissolve in water)-use porous filter to separate the solids out. (If the pores are smaller than the solids)
-use filter paper-residue left in filter paper, filtrate, filtrate goes through filter paper
Crystallization(Solid in liquid)-precipitation is the conversion of a solution to solid form by chemical or physical change
-solids are then separated by filtration or floatation
-saturated solution of a desire solid
-evaporate or cool-solids come out as pure crystals. Then crystals are filtered from remaining solvent.
Gravity Separation-solids based on density
-A centrifuge whirls the test tube around at high speed forcing the denser materials to the bottom. Work best for small volumes.
Solvent Extraction-a component moves into a solvent shaken with the mixture
-works best with solvents that dissolve only one components
-mechanical mixture:(Solid & Solid)
2 solids use liquid, one dissolved, get another one.
-solution: solvent is insoluble with solvent already present.
solvent dissolved 1 or more desire solids and leaves unwanted solids behind.
(If shaken in a separator funnel, the liquids will form layers then drain the solvent to leave the wanted material)
Distillation(Liquid in liquid solution)-heating a mixture can cause low-boiling components to volatilize(vaporize)
-distillation is collecting and condensing volatilized components
-liquids with lowest boiling temperature boil first---vapor ascents to distillation flask and enters condenser; gas cools and condenses back to liquid dropping the distillate as a purified liquid.
Chromatography-flow the mixture over a material that retains some components more than others, so different components flow over the material at different speeds.
-a mobile phase sweeps the sample over a stationary phase
-can separate very complex mixtures
-very small sample sizes analyses----highly accurate & precise
-separated components can be collected individually
Paper Chromatography(PC):-stationary phase is liquid soaked into a sheet or strip paper mobile phase is a liquid solvent some components spend more time in stationary phase than other components appear as separate spots spread out on the paper after drying or "developing".
Sheet Chromatography:Thin layer chromatography (TLC):-stationary phase is a thin layer of absorbent (Al202 or So2 usually) coating a sheet of plastic or glass some components bond to the absorbent strongly other more weakly as with paper chromatography, components appear as spots on the sheet.
-the more similar the properties are, the more difficult it is to separate them.
-Strategy: use any possible way to discriminate between components with different properties.
Hand Separation & Evaporation:-Hand separation (Solid+Solid)
-Use a magnet or sieve
-Evaporation(Solid dissolved in liquids) boil away the liquid and the solids remain
Filtration (Solids NOT dissolve in water)-use porous filter to separate the solids out. (If the pores are smaller than the solids)
-use filter paper-residue left in filter paper, filtrate, filtrate goes through filter paper
Crystallization(Solid in liquid)-precipitation is the conversion of a solution to solid form by chemical or physical change
-solids are then separated by filtration or floatation
-saturated solution of a desire solid
-evaporate or cool-solids come out as pure crystals. Then crystals are filtered from remaining solvent.
Gravity Separation-solids based on density
-A centrifuge whirls the test tube around at high speed forcing the denser materials to the bottom. Work best for small volumes.
Solvent Extraction-a component moves into a solvent shaken with the mixture
-works best with solvents that dissolve only one components
-mechanical mixture:(Solid & Solid)
2 solids use liquid, one dissolved, get another one.
-solution: solvent is insoluble with solvent already present.
solvent dissolved 1 or more desire solids and leaves unwanted solids behind.
(If shaken in a separator funnel, the liquids will form layers then drain the solvent to leave the wanted material)
Distillation(Liquid in liquid solution)-heating a mixture can cause low-boiling components to volatilize(vaporize)
-distillation is collecting and condensing volatilized components
-liquids with lowest boiling temperature boil first---vapor ascents to distillation flask and enters condenser; gas cools and condenses back to liquid dropping the distillate as a purified liquid.
Chromatography-flow the mixture over a material that retains some components more than others, so different components flow over the material at different speeds.
-a mobile phase sweeps the sample over a stationary phase
-can separate very complex mixtures
-very small sample sizes analyses----highly accurate & precise
-separated components can be collected individually
Paper Chromatography(PC):-stationary phase is liquid soaked into a sheet or strip paper mobile phase is a liquid solvent some components spend more time in stationary phase than other components appear as separate spots spread out on the paper after drying or "developing".
Sheet Chromatography:Thin layer chromatography (TLC):-stationary phase is a thin layer of absorbent (Al202 or So2 usually) coating a sheet of plastic or glass some components bond to the absorbent strongly other more weakly as with paper chromatography, components appear as spots on the sheet.
Naming ionic, covalant and Acidic
Acids are formed when a compound composed of Hydrogen ions and a negatively charged ion are dissolved in water(aqueous, aq)!-ions separate when dissolved in water
-H+ ions join with H2O from H3O+ (Hydronium ion)
ex: H + Cl --> HCl
HCl(g)+H2O-->H3O(aq)+Cl(aq)
How to name acids?
For Simple Acids:1. use "hydro" as the beginning
2. last syllable of the nonmetal is dropped and replaced with "-ic"
3. add "acid" at the end.
formula:*****ide ===> hydro*****ic acid
ex.HF: hydrofluoric acid
HCl: hydrochloric acid
HBr: hydrobromic acid
HCN: hydrocyanic acid
For Complex Acids:1. -ate replace with "-ic"
-ite replace with "-ous"
2. "Acid" at the end of the name
formula:*****ate ===> *****ic acid
*****ite ===> *****ous acid
ex.HNO3: Nitric acid
HNO2: Nitrous acid
H3PO4: Phosphoric acid
H2SO4: Sulphuric acid (exception)
-H+ ions join with H2O from H3O+ (Hydronium ion)
ex: H + Cl --> HCl
HCl(g)+H2O-->H3O(aq)+Cl(aq)
How to name acids?
For Simple Acids:1. use "hydro" as the beginning
2. last syllable of the nonmetal is dropped and replaced with "-ic"
3. add "acid" at the end.
formula:*****ide ===> hydro*****ic acid
ex.HF: hydrofluoric acid
HCl: hydrochloric acid
HBr: hydrobromic acid
HCN: hydrocyanic acid
For Complex Acids:1. -ate replace with "-ic"
-ite replace with "-ous"
2. "Acid" at the end of the name
formula:*****ate ===> *****ic acid
*****ite ===> *****ous acid
ex.HNO3: Nitric acid
HNO2: Nitrous acid
H3PO4: Phosphoric acid
H2SO4: Sulphuric acid (exception)
Lab---Chemical & Phsical Change
Lab Purpose:
Learning how to infer the reactions are whether chemical changes or physical changes. And record some recongnizable characteristics of chemical changes.
Materials and Equipments:
Refer to page 18 in Health Lab Text , Lab 2C
Equipments:
4 small test tubes 10mmX75mm
test tube rack
4 medicine dropper
glass square
lab apron
safety goggles
four unknown chemical reagents(one is green, the others are all transparent)
procedure:
Refer to Page 18 in Health Lab Text, Lab 2C
Learning how to infer the reactions are whether chemical changes or physical changes. And record some recongnizable characteristics of chemical changes.
Materials and Equipments:
Refer to page 18 in Health Lab Text , Lab 2C
Equipments:
4 small test tubes 10mmX75mm
test tube rack
4 medicine dropper
glass square
lab apron
safety goggles
four unknown chemical reagents(one is green, the others are all transparent)
procedure:
Refer to Page 18 in Health Lab Text, Lab 2C
Analysis of Results
B+A Chemical Change
C+A Physical Change
D+A Chemical Change
C+B Chemical Change
D+B Chemical Change
D+C Physical Change
Following-Up Questions:
a)Chemical change: Burn some papers, cook meat
b)Physical change: tear some papers, ice melt
Sources of Error:
There are some drops of liquid in the glass square.
That could be water or other solutions which might affect the results.
Conclusion:
According to the results of this lab, we conclude that chemical changes happen with new substances formed and physical changes take place with new substances formed.
Not like chemical changes, physical changes are reversable.
In this lab, chemical changes happen in the form of color changing, air bubbles and sediments generation.
Matter & Chemical and Phsical Change
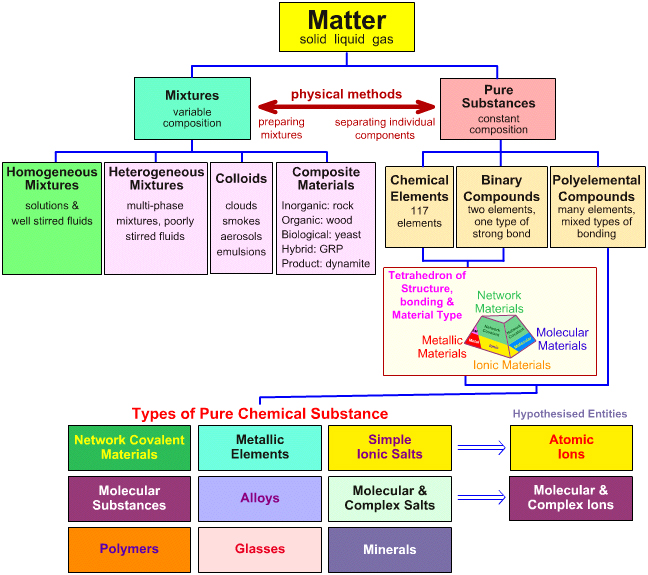
MATTER?
----Anything that has mass and takes up space.
Matter
includes
1.Pure Substances
---one set of properties
---one kind of particle
---simplest form(cannot be decomposed)
---made of atoms 1.metal 2.nonmetal 3.metalloid
(2)compounds----made of elements
---smallest particle is a molecule(Ionic & Covalent)
2.Mixtures
---more than one set of properties and substances
---uniform throughout
---appears to have only one component
Ex: Solutions & colloid
---not uniform---appears to have more than one component
Ex: Salad Dressing (Suspensior & Mechanical Mixture)
Water+oil
Physical Change vs. Chemical Change
Physical Change---no new substance is formed
---chemical composition doesn't change
---reversible
Chemical Change
---new substances are formed
---irreversible
A few more properties for matter
---it is neither created nor destroyed(only changed form from one form to another)
Three States
(1)Solid
(2)Liquid
---takes the shape of the container and experiences slight changes in volume when heated
(3)Gases
---takes the shape of the container and experiences drastic change in volume when heated
2011年10月18日星期二
Unit conversion
Unit Conversion All measurement in science come in two parts: the number and the unit, having one without the other makes no sense!
SI Metric:
1. SI stands for systeme internationale
2. It is a French system dating back to the early 1800s
3. It uses powers of 10!
The Canceling Method for unit conversions:
Derived quantities include units created by combining base quantities through multiplication or division
ex. m^2(m*m)——Area
cm^3 (cm*cm*cm)——Volume
km/h or m/s——speed
g/L ——density
ex. m^2(m*m)——Area
cm^3 (cm*cm*cm)——Volume
km/h or m/s——speed
g/L ——density
订阅:
评论 (Atom)



