-Functional Groups-
Functional Groups: Specific group of atoms whihch exists in a molecule and gives a specific properties to a molecule.
Ex: They can lead the molecule into many different other form such as acid, base or both
-Here is some example of Functional Group-
A.) Alcohols
-Alcohol contains OH in the compound (Organic Compound ofcourse~)
-For example: CH3-OH, CH3-CH2-OH
-Alcohol is soluable in water and non-polar hydrocarbon chain tends to make alcohols insoluable in water
-All alcohols are poisonous (So don't drink alcohol too much~You will die!)
B.) Aldehyde
-Aldehyde has C=O group at the end of the hydrocarbon chain
-Aldehyde can simply look like this: -CHO
C.) Ketones
-Ketone is organic compound that have C=O group similar to Aldehyde but not at the end of the Hydrocarbon Chain
D.) Ethers
-Ether is compound in which an oxygen joins two hydrocarbon groups
-Fact: Ehers can be act as anaesthetic purposes
Ex of Ethers: CH3CH2-O-CH2CH3
E.) Amines
-Amine is an organic compound containing an NH2 group
-Amine is basic and reacts with acid
-It has fishy-like odour (Which means it smells horrible)
F.) Amide
-Amide is an organic compound containing a CONH2 Group
G.) Carboxylic Acid
-Carboxylic Acid has COOH group.
-Also refered to as Organic Acids
H.) Esters (Hm? Don't we have name Ester in school or something?)
-Ester is compound that has COO group which joins two hydrocarbon chains
R
2012年5月28日星期一
2012年5月22日星期二
Aldehydes and Ketones
- is an organic compound that contains a double bond of carbon and oxygen and a single alkyl substitute
- the ending of the aldehyde is written as “-al”
Ketones
- is an organic compound that contains a double bond of carbon and oxygen as well as two alkyl substitutes
- Its general formula would be CnH2nO where n represent the number of carbons in the structure
- the ending of ketone is written as “-one”
Ex 1.
2. the double bond of carbon and oxygen is located at the first carbon
3. ending is “-al”
Name of Structure: butanal
Ex 2.
Draw the structure methanol
1. methyl is one carbon
2.double bond is located at the first carbon
Ex 3.
2. the double bond of carbon and oxygen is located at the second carbon
3. nitro is located 4th from the left side
4. ending is “-al”
Name of Structure: 4-nitro-2-pentanone
Ex 4.
Draw the structure 3-ethyl-3-hexenal
1. hexane is a 7 carbon chain
2. double bond is located on the 3rd carbon
3. ethyl is located on the 3rd carbon
4. ending “-al” signifies there is a double bond of oxygen and carbon
2012年5月10日星期四
Alkanes and Alkynes
Alkanes and alkynes are just like organic compounds but the carbons in the molecular structure will most likely form double and triple bonds

Ex 1. CH2 = C = CH2 double bonds in the naming is signified by putting the placement in front of the carbon chain which is at the end. In this structure there are two double bonds, one and 1 and the other at 2, with a carbon pain of 3.
1,2 – propene the ending for propane is changed to ene to signify that there is a double bond
Ex 2. CH3
l
CH3 – CH – CH = C = CH2
The longest carbon chain is 5, double bonds are located first and second, and a branch is 4 from the most left point, with one carbon in the branch.
Name of the structure: 4 – methyl – 1,2 – pentene
Ex 3. CH3
l
CH3 – C = C – CH2 – CH3
l
CH3
The longest carbon chain is 5, double bond is located on the third, and there are two branches, one on the third and fourth from the most right with one carbon on each branch
Name of the structure: 4 – methyl – 3 – methyl – 3 – pentene
Chemistry Music Video 29: It's A Family Thing
Alkenes-Alkynes
Satannia 8
Organic Compounds
Organic compounds will most likely contain carbon

An example would be cocaine, C17H21NO4
Methane is the simplest of the organic compounds
A homologous series consist of methane, ethane, propane, butane, pentane, hexane, heptanes, octane, nonane and decane
Homologous means that they have similar formula
Some organic compounds may not consist of hydrogen and carbon, examples would include tetrachloride
A alkene is a bond of hydrogen and carbon, in which that all carbon atoms are bonded by single bonds of hydrogen to make octets
Ex 1. H H H H H H H H
l l l l l l l l
H – C – C – C – C – C – C –C – C – H
l l l l l l l l
H H H H H H H H
This would be an octane as the longest chain of carbon is 8
These are the basic steps to take in naming an organic compound molecular structure (steps from Ms.Chen)
Find and name the longest continuous carbon chain that will be the name for the end.
Depending on the number of carbons the name would be: Methane – 1
Ethane – 2
Propane – 3
Butane – 4
Pentane – 5
Hexane – 6
Heptane – 7
Octane – 8
Nonane – 9
Decane – 10
Identify and name groups attached to chains.
Depending on the number of carbon that branch off that would make up the second part in which if there is one carbon on a branch that would give it the name methyl. It is basically similar to the top but the ane becomes yl. The number that comes before the group name is the position of the branch.
Putting the groups in alphabetical order and the carbon chain at the end it the complete name of the molecular structure
Ex 2. CH3
l
CH3 – C – CH3 Longest carbon chain is 3, number of carbon in branch is 1 and
l branches are found 2 to the most left
CH3 2,2 – methyl – propane
CPR Episode 1 - Organic Chemistry Nomenclature
OChem 01 - Nomenclature 1/4
Satannia 8
2012年4月24日星期二
Structure of the atom

- The structure of the atom contains 3 small particles that each have their own charge
- In the center of the atom there are protons and neutrons
- Protons has a +1 charge where else neutrons have a 0 charge
- Outside of the nucleus is the electron with their -1 charge
- Usually in an atom the number of protons equal to the number of electrons
- is commonly the symbol for electron, symbolizes proton and symbolizes neutrons
- Number of neutrons is commonly calculated using the difference of the atomic mass and the number of protons
- Electrons are always on the outside and circle the nucleus in their corresponding valence shell
History of the atom
- It had all started off with Aristotle’s theory that everything was made up of an even smaller particle.
 | ||||
Aristotle
|
2012年4月23日星期一
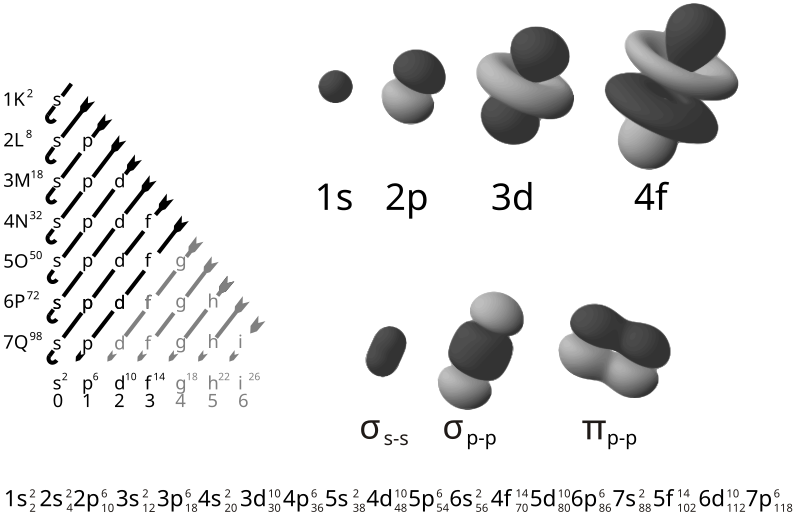
Electron Configuration and Valence Electrons
Electron Configuration
definition: A statement describing the populations of electronic energy sublevels of an atom. See the chart of electronic configurations to get the notation for all of the elements.
example: The electronic configuration of the lithium atom is 1s22s, which indicates there are two elements in the 1s sublevel and one electron in the 2s energy sublevel
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule in atomic or molecular orbitals.
According to the laws of quantum mechanics, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one orbital to another by emission or absorption of a quantum of energy, in the form of a photon.
definition: An electron in an outer shell of an atom that can participate in forming chemical bonds with other atoms
2012年3月8日星期四
Lab 6D
Determining the Limiting Reactant and Percent Yield in a Precipitation Reaction.
The OBJECTIVES of this lab are to determine which of the reactants is the limiting reactant and which is the excess reactant; to determinr the theoretical mass of precipitate that should form; to compare the actual mass with the theoretical mass of precipitate and calculate.
The equipment we used in this lab is a little different form before, we use filtering apparatus and filter paper to filtrate the products.
There is a small tip when using filter paper: we'd better use water to wet the paper in order to let it accrete on the filtering qpparatus.
Chemical reagents we used in this lab are 25ml 0.70M sodium carbonate solution, Na2CO3 and 25ml 0.50M calcium chloride solution, CaCl2.
Na2CO3(aq) + CaCl2(aq) = 2NaCl(aq) + CaCO3 (s)
(CaCO3 is white solid)
0.7M * 0.025L*(1 mol NaCO3/1 mol CaCO3) * (100g/mol) = 1.75g CaCO3
0.5M * 0.025L*(1 mol CaCl2/ 1 mol CaCO3) *(100g/mol) = 1.25g CaCO3
Na2CO3 will be left!
So that after filtrate the solid is CaCO3, the solution is Na2CO3+NaCL
After measure, we find the product we get actually is 1.02g
% Yield = 1.02g/1.25g *100% = 0.82%
The OBJECTIVES of this lab are to determine which of the reactants is the limiting reactant and which is the excess reactant; to determinr the theoretical mass of precipitate that should form; to compare the actual mass with the theoretical mass of precipitate and calculate.
The equipment we used in this lab is a little different form before, we use filtering apparatus and filter paper to filtrate the products.
There is a small tip when using filter paper: we'd better use water to wet the paper in order to let it accrete on the filtering qpparatus.
Chemical reagents we used in this lab are 25ml 0.70M sodium carbonate solution, Na2CO3 and 25ml 0.50M calcium chloride solution, CaCl2.
Na2CO3(aq) + CaCl2(aq) = 2NaCl(aq) + CaCO3 (s)
(CaCO3 is white solid)
0.7M * 0.025L*(1 mol NaCO3/1 mol CaCO3) * (100g/mol) = 1.75g CaCO3
0.5M * 0.025L*(1 mol CaCl2/ 1 mol CaCO3) *(100g/mol) = 1.25g CaCO3
Na2CO3 will be left!
So that after filtrate the solid is CaCO3, the solution is Na2CO3+NaCL
After measure, we find the product we get actually is 1.02g
% Yield = 1.02g/1.25g *100% = 0.82%
Percent Yield and Purity
 From last class, we knew that in real chemical reactions, chemicals are not always able to be recoverd, or some reactants will not use up. In that case, we are not possible to get same amount of products as we want according to equations.
From last class, we knew that in real chemical reactions, chemicals are not always able to be recoverd, or some reactants will not use up. In that case, we are not possible to get same amount of products as we want according to equations.So that we have to calculate % Yield!!!
% Yield = (grams of actual product recoverd) / (grams of products expected for stoich) * 100%
We also know that not all the chemicals are pure,
for instance, most of the metal in earth are exist in type of alloy.
So that we have to calculate % Purity before we solve the problems
% Purity = (Mass of pure substance) / (Mass of Impure sample) * 100%

Example: 0.1g H2 appears when sufficient Iron react with 100g HCl which the purity is 12%, what is the % Yield?
First, balance equation: Fe + 2HCl = FeCl2 + H2
100g * 12% / (36.5g/mol) * (1 mol H2/ 2 mol HCl) * 2g/ mol = 0.33g H2
% Yield = 0.1g / 0.33g * 100% = 30.3%
Excess & Limiting reactant
Excess quantity: In real chemical reactions, it is necessary to add more of the reactan than the equation predicts because it is not possible for every atom/molecular of reactants to come together.
One reactant is the excess quantity and some of it will be left over. The second reactant is used up completely.

When dealing with this kind of problems, having a correctly balanced equation is always important.
Because it will give a prediction and with that, you can find how much chemical left.
Example: Which chemical will left when 20g Mg react with 10g HCl and how much will left?
First, give a correctly balanced equation: Mg +2 HCl == MgCl2 + H2
1 mol Mg can make 1 mol H2;
2 mol HCl can make 1 mol H2.
20g/(24.3g/mol)*(1 mol H2/ 1 mol Mg) = 0.82 mol H2 = 1.64g H2
10g/(36.5g/mol)*(1 mol H2/2 mol HCl) = 0.14 mol H2= 0.28g H2
At that time, we can clearly see that 20g Mg can make more H2, so that Mg will be left and O2 is going to be used up.
But how much Mg will left?
0.14 mol H2 * (1 mol Mg/ 1 mol H2) * 24.3g/mol = 3.4g
20g-3.4g = 16.6 g Mg left
One reactant is the excess quantity and some of it will be left over. The second reactant is used up completely.

When dealing with this kind of problems, having a correctly balanced equation is always important.
Because it will give a prediction and with that, you can find how much chemical left.
Example: Which chemical will left when 20g Mg react with 10g HCl and how much will left?
First, give a correctly balanced equation: Mg +2 HCl == MgCl2 + H2
1 mol Mg can make 1 mol H2;
2 mol HCl can make 1 mol H2.
20g/(24.3g/mol)*(1 mol H2/ 1 mol Mg) = 0.82 mol H2 = 1.64g H2
10g/(36.5g/mol)*(1 mol H2/2 mol HCl) = 0.14 mol H2= 0.28g H2
At that time, we can clearly see that 20g Mg can make more H2, so that Mg will be left and O2 is going to be used up.
But how much Mg will left?
0.14 mol H2 * (1 mol Mg/ 1 mol H2) * 24.3g/mol = 3.4g
20g-3.4g = 16.6 g Mg left
Stoichiometry calculation
In summary, stoichiometry is the study of quantitive aspect of chemical reaction.
When dealing with the stoichiometry calculations, first of all, you have to get a correctly balanced equation. The number in front of each reactants and products means the quantity of each chemicals in the reaction. Then, we can solve the Stoichiometry problems through the ratio of each chemicals.

Example : How many moles of H2 can we get when 20g Iron react with sufficient HCl?
First, we write the eaqution and balance it:
1 Fe(s) + 2 HCl(aq) == 1 FeCl2(aq) + 1 H2(gas)
Second, we change 20g Iron into moles:
the mole mass of iron is 56g/mol.
20g/(56g/mol)=0.36mol iron
according to the banlanced eqution we made before: 1 mol Fe can make 1 mole H2
So that 0.36 mol/ (1 mol H2/ 1 mol Fe)= 0.36mol H2
We can get 0.36 mol H2 when 20g Iron react with sufficient HCl
Hint: through the balanced equation, we can directly get the ratio of Fe and H2= 1:1.
When dealing with the stoichiometry calculations, first of all, you have to get a correctly balanced equation. The number in front of each reactants and products means the quantity of each chemicals in the reaction. Then, we can solve the Stoichiometry problems through the ratio of each chemicals.

Example : How many moles of H2 can we get when 20g Iron react with sufficient HCl?
First, we write the eaqution and balance it:
1 Fe(s) + 2 HCl(aq) == 1 FeCl2(aq) + 1 H2(gas)
Second, we change 20g Iron into moles:
the mole mass of iron is 56g/mol.
20g/(56g/mol)=0.36mol iron
according to the banlanced eqution we made before: 1 mol Fe can make 1 mole H2
So that 0.36 mol/ (1 mol H2/ 1 mol Fe)= 0.36mol H2
We can get 0.36 mol H2 when 20g Iron react with sufficient HCl
Hint: through the balanced equation, we can directly get the ratio of Fe and H2= 1:1.
2012年2月15日星期三
Stochiometry
- Stoichiometry is used to measure the total amount of substances in a chemical reaction
- it relates to the law of conservation of mass and the law of definite proportions
- used to determine the amount of reactant and product in the chemical reaction
- when using stoichiometry a balanced equation is needed in order to calculate the correct answer
ex 1. With 27.1g of calcium oxide, what is the amount of moles of calcium oxide.
27.1g CaO*(1 mol CaO/56.08g CaO)= 0.484 mol CaO
ex 2. What is the total amount of sodium hydroxide that is needed to make 250 ml of 0.200M solution, in grams.
stoichiometry tutorial
steps to solving stoichiometric problem
mole-to-mole and mass-to-mass conversions
- it relates to the law of conservation of mass and the law of definite proportions
- used to determine the amount of reactant and product in the chemical reaction
- when using stoichiometry a balanced equation is needed in order to calculate the correct answer
ex 1. With 27.1g of calcium oxide, what is the amount of moles of calcium oxide.
27.1g CaO*(1 mol CaO/56.08g CaO)= 0.484 mol CaO
ex 2. What is the total amount of sodium hydroxide that is needed to make 250 ml of 0.200M solution, in grams.
0.500L NaOH*(0.200mol NaOH/ 1L)*(40.0g NaOH/1mol NaOH)= 4.02g
stoichiometry tutorial
steps to solving stoichiometric problem
mole-to-mole and mass-to-mass conversions
2012年2月13日星期一
Lab 5B
Lab 5B is the first lab we did which dealing with reaction this term. There are totally seven reactions including Sythesis, Single Replacement, Decomposition and Double Replacement. The objective of this lab is to observe a varity of chemical reactions and to classify each reaction as one of the four main types.
(Several chemical reagents have been used in this lab, so wearing a safety goggle and lab apron is necessary. )
Reaction 1 (2 Cu + O2 = 2 CuO)is a sythesis reaction, though it need high temperature to achieve the reaction. In that case, you will find red copper wire become dark black at last.
Reaction 2 (2 Fe +3 CuSO4 = Fe2(SO4)3 + 3Cu) is a Single Replacement, which using Fe to replace Cu. We put a iron nail into blue solution which is CuSO4. Several seconds later, some rust appears and the shiny iron nail becomes black.
Reaction 7 (2 H2O2 + (MnO2) = 2 H2O + O2). This one is quite different from before, in that case, MnO2 is a kind of catalyzer which will not avolved in the reaction. So as you see in right hand side, the products, there is even no element Mn. However, this catalyzer is quite good, the reaction will be very fast with only a little MnO2.
This lab shows some basic reactions in daily chemistry. Some reactions are very familiar in our everyday life. For example, reaction 3(CuSO4*5H2O = CuSO4 + 5 H2O) is the most common way to make pure CuSO4 in industry.
(Several chemical reagents have been used in this lab, so wearing a safety goggle and lab apron is necessary. )
Reaction 1 (2 Cu + O2 = 2 CuO)is a sythesis reaction, though it need high temperature to achieve the reaction. In that case, you will find red copper wire become dark black at last.
Reaction 2 (2 Fe +3 CuSO4 = Fe2(SO4)3 + 3Cu) is a Single Replacement, which using Fe to replace Cu. We put a iron nail into blue solution which is CuSO4. Several seconds later, some rust appears and the shiny iron nail becomes black.
Reaction 7 (2 H2O2 + (MnO2) = 2 H2O + O2). This one is quite different from before, in that case, MnO2 is a kind of catalyzer which will not avolved in the reaction. So as you see in right hand side, the products, there is even no element Mn. However, this catalyzer is quite good, the reaction will be very fast with only a little MnO2.
This lab shows some basic reactions in daily chemistry. Some reactions are very familiar in our everyday life. For example, reaction 3(CuSO4*5H2O = CuSO4 + 5 H2O) is the most common way to make pure CuSO4 in industry.
Balancing equation
Balancing equation is like a math work. It is depended on The law of conservation of mass. So that, the mass of reactants = the mass of products.
When balancing a equation, first you have to count the number of atoms of each elements.
For example, H2+O2=H2O, there are 2 H and 2 O in left hand side and there are 2 H and 1 O in right hand side. That means H:O=2:1. So for reactants H:O should be 2:1 too. So there should be 4H and 2O in right hand side and left hand side. Finally, we get 2H2+2O2=2H2O....
For some other types of reactions like single replacement, you can use a easier way to balance equation. For example, Fe + CuSO4 = Cu + Fe2(SO4)3. In that case, (SO4)2- can be seen as a whole. So you can find there are 2 (SO4)2- in right hand side. Than you can guess 3 CuSO4 in left hand side, that means there should be 3 Cu in right hand side. At last, obviously, 2 Fe in both right and left hand side. Finally the equation should be 2 Fe + 3 CuSO4 = 3 Cu + Fe2(SO3)2.
That is a my own way to balance equation. You may find a Better way when doing more exercise. Many convinience strategy will be found when you get more experience in balancing equation.
When balancing a equation, first you have to count the number of atoms of each elements.
For example, H2+O2=H2O, there are 2 H and 2 O in left hand side and there are 2 H and 1 O in right hand side. That means H:O=2:1. So for reactants H:O should be 2:1 too. So there should be 4H and 2O in right hand side and left hand side. Finally, we get 2H2+2O2=2H2O....
For some other types of reactions like single replacement, you can use a easier way to balance equation. For example, Fe + CuSO4 = Cu + Fe2(SO4)3. In that case, (SO4)2- can be seen as a whole. So you can find there are 2 (SO4)2- in right hand side. Than you can guess 3 CuSO4 in left hand side, that means there should be 3 Cu in right hand side. At last, obviously, 2 Fe in both right and left hand side. Finally the equation should be 2 Fe + 3 CuSO4 = 3 Cu + Fe2(SO3)2.
That is a my own way to balance equation. You may find a Better way when doing more exercise. Many convinience strategy will be found when you get more experience in balancing equation.
2012年2月12日星期日
Endothermic/ Exothermic
Endothermic and Exothermic
endothermic: reaction in which the system absorbs energy from the surroundings in the form of heat.
exothermic:a chemical reaction that releases energy in the form of light or heat
endothermic: reaction in which the system absorbs energy from the surroundings in the form of heat.
exothermic:a chemical reaction that releases energy in the form of light or heat
type of reactions
Chemical reactions can be classified as one of six main types: synthesis, decomposition, single replacement, double replacement, neutralization, combustion.
SYNTHESIS: A synthesis reaction is when two or more simple compounds combine to form a more complicated one.
element+element--compound
A+B --AB
Eg: hydrogen + oxygen -- water
DECOMPOSITION: A decomposition reaction is the opposite of a synthesis reaction - a complex molecule breaks down to make simpler ones
compound--element+element
AB--A+B
Eg: calcium chlorate-- calcium chloride + oxygen
SINGLE REPLACEMENT: This is when one element trades places with another element in a compound.
element+compound --element + compound
A+BC--B+AC [A=metal]
A+BC--C+BA [A= non metal]
Eg aluminum+ lead nitrate--aluminum nitrate +lead
DOUBLE REPLACEMENT : This is when the anions and cations of two different molecules switch places, forming two entirely different compounds
ionic solution+ ionic solution-- ionic solution + ionic solid
AB[aq] +CD[aq]--AD[aq]+CB[s]
Eg: iron chloride + lithium phosphate--iron phosphate + lithium chloride
NEUTRALIZATION :This is a special kind of double displacement reaction that takes place when an acid and base react with each other.
acid+ base--salt+water
HA+BOH---AB +H2O
Eg; sulphuric acid + sodium hydroxide--sodium sulphate +water
COMBUSTION: A combustion reaction is when oxygen combines with another compound to form water and carbon dioxide.
Eg: hydrocarbon+ oxygen --carbon dioxide +water
SYNTHESIS: A synthesis reaction is when two or more simple compounds combine to form a more complicated one.
element+element--compound
A+B --AB
Eg: hydrogen + oxygen -- water
DECOMPOSITION: A decomposition reaction is the opposite of a synthesis reaction - a complex molecule breaks down to make simpler ones
compound--element+element
AB--A+B
Eg: calcium chlorate-- calcium chloride + oxygen
SINGLE REPLACEMENT: This is when one element trades places with another element in a compound.
element+compound --element + compound
A+BC--B+AC [A=metal]
A+BC--C+BA [A= non metal]
Eg aluminum+ lead nitrate--aluminum nitrate +lead
DOUBLE REPLACEMENT : This is when the anions and cations of two different molecules switch places, forming two entirely different compounds
ionic solution+ ionic solution-- ionic solution + ionic solid
AB[aq] +CD[aq]--AD[aq]+CB[s]
Eg: iron chloride + lithium phosphate--iron phosphate + lithium chloride
NEUTRALIZATION :This is a special kind of double displacement reaction that takes place when an acid and base react with each other.
acid+ base--salt+water
HA+BOH---AB +H2O
Eg; sulphuric acid + sodium hydroxide--sodium sulphate +water
COMBUSTION: A combustion reaction is when oxygen combines with another compound to form water and carbon dioxide.
Eg: hydrocarbon+ oxygen --carbon dioxide +water
2012年2月8日星期三
-energy absorption and reduction may be laced in the equation
-exothermic reaction have the Right hand side energy term and is usually a negative ΔH
-endothermic reaction have the left hand side energy term and is usually a positive ΔH
- ΔH=energy change of reaction and expressed in kJ/mol of one of the chemical
Eg. 2Ba+O2→2BaO+1115kJ
for this equation the exothermic reaction would be 557.5kJ/1mol Ba or 1115kJ/2mol O2
-ΔH value depends on which chemical is used in the equation
-molar mass is different for each chemical reaction, so is the ΔH for different chemical reaction
2Ba+O2→2BaO+1115kJ
-1500kJ*-1molBa/1115kJ= 1.345mol Ba= 1.3mol Ba
Example 1:
2012年1月10日星期二
Atomic/ Formular/ Molecular/ Molar mass
Atom is the smallest particle of a chemical element that retains its chemical properties.
A compound's empirical formula is the simplest integer ratio of the chemical elements that constitute it. For example, water is always composed of a 2:1 ratio of hydrogen to oxygen atoms, and ethyl alcohol or ethanel is always composed of carbon, hydrogen, and oxygen in a 2:6:1 ratio. However, this does not determine the kind of molecule uniquely – dimethyl ether has the same ratios as ethanol, for instance. Molecules with the same atoms in different arrangements are called isomers. Also carbohydrates, for example, have the same ratio (carbon:hydrogen:oxygen = 1:2:1) but different total numbers of atoms in the molecule.
The molecular formula reflects the exact number of atoms that compose the molecule and so characterizes different molecules. However different isomers can have the same atomic composition while being different molecules.
The empirical formula is often the same as the molecular formula but not always. For example, the molecule acetylene has molecular formula C2H2, but the simplest integer ratio of elements is CH.
The molecular mass can be calculated from the chemical formula and is expressed in conventional atomic mass units equal to 1/12 of the mass of a neutral carbon-12 atom. For network solids, the term formul unit is used in stoichiometric calculations.
In order to grasp the concept of molar mass calculations it is important to understand the molar unit. The mole also called mol is the basic unit of measurement in chemistry. By definition, in modern chemistry, one mole represents the number of carbon atoms in exactly 12 grams of carbon 12 isotope. Remember that carbon-12 has an atomic mass of 12 (six neutrons and six protons).
One mole of anything, however, contains 6.0221367E23 of that object. This is known as Avogadro's number.
Examples:
1 mole of carbon = 6.0221367E23 carbon atoms 1 mole of bananas = 6.0221367E23 bananas
A compound's empirical formula is the simplest integer ratio of the chemical elements that constitute it. For example, water is always composed of a 2:1 ratio of hydrogen to oxygen atoms, and ethyl alcohol or ethanel is always composed of carbon, hydrogen, and oxygen in a 2:6:1 ratio. However, this does not determine the kind of molecule uniquely – dimethyl ether has the same ratios as ethanol, for instance. Molecules with the same atoms in different arrangements are called isomers. Also carbohydrates, for example, have the same ratio (carbon:hydrogen:oxygen = 1:2:1) but different total numbers of atoms in the molecule.
The molecular formula reflects the exact number of atoms that compose the molecule and so characterizes different molecules. However different isomers can have the same atomic composition while being different molecules.
The empirical formula is often the same as the molecular formula but not always. For example, the molecule acetylene has molecular formula C2H2, but the simplest integer ratio of elements is CH.
The molecular mass can be calculated from the chemical formula and is expressed in conventional atomic mass units equal to 1/12 of the mass of a neutral carbon-12 atom. For network solids, the term formul unit is used in stoichiometric calculations.

In order to grasp the concept of molar mass calculations it is important to understand the molar unit. The mole also called mol is the basic unit of measurement in chemistry. By definition, in modern chemistry, one mole represents the number of carbon atoms in exactly 12 grams of carbon 12 isotope. Remember that carbon-12 has an atomic mass of 12 (six neutrons and six protons).
One mole of anything, however, contains 6.0221367E23 of that object. This is known as Avogadro's number.
Avogadro's number and hypohesis
Avogadro's number, also known as Avogadro's constant, is defined as the quantity of atoms in precisely 12 grams of 12C. The designation is a recognition of Amedeo Avogadro, who was the first to state that a gas' volume is proportional to how many atoms it has. Avogadro's number is given as 6.02214179 x 10-23 mol.
Amadeo Avogadro lived in the early 19th century, and was an Italian savant known for his role in many different scientific disciplines. His most famous statement is known as Avogadro's Law, and is a hypothesis that states: “Equal volumes of ideal or perfect gasses, at the same temperature and pressure, contain the same number of particles, or molecules.”
This is an intriguing hypothesis, because it says that quite different elements, such as nitrogen and hydrogen, still have the same number of molecules in the same volume of an ideal gas. While in real world settings this is not strictly true, it is statistically quite close, and so the ideal model still has a great deal of value.

Amadeo Avogadro lived in the early 19th century, and was an Italian savant known for his role in many different scientific disciplines. His most famous statement is known as Avogadro's Law, and is a hypothesis that states: “Equal volumes of ideal or perfect gasses, at the same temperature and pressure, contain the same number of particles, or molecules.”
This is an intriguing hypothesis, because it says that quite different elements, such as nitrogen and hydrogen, still have the same number of molecules in the same volume of an ideal gas. While in real world settings this is not strictly true, it is statistically quite close, and so the ideal model still has a great deal of value.
Lab 4C
Objectives:
1. To determine the % of water in a unknown hydrate.
2. To determine the moles of water present in each mole of this unknown hydrate, when give the molar mass of the anhydrous salt.
3. To write the empirical formular of the hydrate.
Supplies:
lab burnet, ring stand and ring, crucible and lid, centigram or digital balance, crucible tongs, pipestem triangle, lab apron, safety goggles.
Chemical Reagents: approximately 5g of hydrate, water.
ProcedureMass a crucible and its cover and record in a data table. Place about 2 grams of the hydrate in the crucible and record the mass of the crucible, cover and hydrated salt. Gently heat the crucible and its contents for about 10 minutes. During the last minute of heating remove the cover so that any moisture which has collected on the underside of the cover can evaporate. When the crucible has cooled determine the mass of the crucible, cover, and contents.
Replace the cover and heat the crucible for five more minutes removing the cover during the last minute of heating as you did above. Cool and determine the mass of the crucible, cover, and contents. This last mass should agree with the previous mass to within plus or minus 0.005 grams. If it does not repeat this heating until a constant mass is reached. This is called heating to a constant mass and is the only way of insuring that the reaction is complete.
Data:
Mass of empty crucible and lid 13.12g
Mass of crucible, lid and hydrate 15.34g
Mass of hydrate 2.22g
Mass of crucible, lid and anhydrous salt(first) 14.54g
Mass of crucible, lid and anhydrous salt(second) 14.55g
Mass of anhydrous salt 1.42g
Mass of water given off 0.82g
Mass of one mole of hydrous salt(from your instructor) 159.6g/mol
Describle any changes that you observed when adding water to the crucible Become blue solid.

1. To determine the % of water in a unknown hydrate.
2. To determine the moles of water present in each mole of this unknown hydrate, when give the molar mass of the anhydrous salt.
3. To write the empirical formular of the hydrate.
Supplies:
lab burnet, ring stand and ring, crucible and lid, centigram or digital balance, crucible tongs, pipestem triangle, lab apron, safety goggles.
Chemical Reagents: approximately 5g of hydrate, water.
ProcedureMass a crucible and its cover and record in a data table. Place about 2 grams of the hydrate in the crucible and record the mass of the crucible, cover and hydrated salt. Gently heat the crucible and its contents for about 10 minutes. During the last minute of heating remove the cover so that any moisture which has collected on the underside of the cover can evaporate. When the crucible has cooled determine the mass of the crucible, cover, and contents.
Replace the cover and heat the crucible for five more minutes removing the cover during the last minute of heating as you did above. Cool and determine the mass of the crucible, cover, and contents. This last mass should agree with the previous mass to within plus or minus 0.005 grams. If it does not repeat this heating until a constant mass is reached. This is called heating to a constant mass and is the only way of insuring that the reaction is complete.
Data:
Mass of empty crucible and lid 13.12g
Mass of crucible, lid and hydrate 15.34g
Mass of hydrate 2.22g
Mass of crucible, lid and anhydrous salt(first) 14.54g
Mass of crucible, lid and anhydrous salt(second) 14.55g
Mass of anhydrous salt 1.42g
Mass of water given off 0.82g
Mass of one mole of hydrous salt(from your instructor) 159.6g/mol
Describle any changes that you observed when adding water to the crucible Become blue solid.

Molarity
Another way of expressing concentration, the way that we will use most in this course, is called molarity. Molarity is the number of moles of solute dissolved in one liter of solution. The units, therefore are moles per liter, specifically it's moles of solute per liter of solution.
Rather than writing out moles per liter, these units are abbreviated as M or M. We use a capital M with a line under it or a capital M written in italics. So when you see M or M it stands for molarity, and it means moles per liter (not just moles).
You must be very careful to distinguish between moles and molarity. "Moles" measures the amount or quantity of material you have; "molarity" measures the concentration of that material. So when you're given a problem or some information that says the concentration of the solution is 0.1 M that means that it has 0.1 mole for every liter of solution; it does not mean that it is 0.1 moles. Please be sure to make that distinction.
Rather than writing out moles per liter, these units are abbreviated as M or M. We use a capital M with a line under it or a capital M written in italics. So when you see M or M it stands for molarity, and it means moles per liter (not just moles).
You must be very careful to distinguish between moles and molarity. "Moles" measures the amount or quantity of material you have; "molarity" measures the concentration of that material. So when you're given a problem or some information that says the concentration of the solution is 0.1 M that means that it has 0.1 mole for every liter of solution; it does not mean that it is 0.1 moles. Please be sure to make that distinction.
All types of mole conversions
1.Conversions PARTICLE/ATOM/FORMULA UNIT-MOLES
2.Comversions MOLES-PARTICLES/ATOMS/FORMULA UNITS
3.Conversions MOLES-GRAMS
Molar mass of C= 12.0g/mole
2.04mol C x 12.0g C/1mol C= 24.5gC
4.Conversion GRAMS-MOLES
Molar mass of C =12.0g/mol
3.45g C x 1mol C/ 12.0g C= 0.287mol C
2 Cl x 35.5u =71.5u/95.3u
Molar mass of Mgcl2 = 95.3g/mol
6.2 g Mgcl2 x 1mol MgCl2/ 95.3gMgCl 2= 0.065 mol MgCl 2
- Example; How many moles of Carbon atoms are there in 3.01x10^24C atoms?
2.Comversions MOLES-PARTICLES/ATOMS/FORMULA UNITS
- Example: How many CO2 molecules are present in 0.75 moles of CO2?
- Example : How many atoms of Oxygen are present in 0.75 mol CO2?
3.Conversions MOLES-GRAMS
- Example: What is the mass in grams of 2.04 moles of Carbon?
Molar mass of C= 12.0g/mole
2.04mol C x 12.0g C/1mol C= 24.5gC
4.Conversion GRAMS-MOLES
- Example: How many moles are there in 3.45 grams of Carbon?
Molar mass of C =12.0g/mol
3.45g C x 1mol C/ 12.0g C= 0.287mol C
- Example: How many moles are there in 6.2 grams of Magnesium chloride?
2 Cl x 35.5u =71.5u/95.3u
Molar mass of Mgcl2 = 95.3g/mol
6.2 g Mgcl2 x 1mol MgCl2/ 95.3gMgCl 2= 0.065 mol MgCl 2
2012年1月5日星期四
Diluting Solution to Prepare Workable Solution
- chemicals are typically shipped around the world in their most concentrated form
- thus making it easier to transport and cost fairly less money
- but have to make concentrate form from any solution from an even more
concentrated solution
Example 1: You have 2.00L of 16.0M HCl, but you need 0.800L of 2.00M HCl
How do you make it?
- numbers of moles are always constant=not changing
- only difference between the two would be there is more water in the less
concentrated solution
Therefore, Equation would be= Moles solute before=Moles solute after
M1L1=M2L2
The subscript 1 denotes “before” and subscript is “after”
Solving for L1 wouls solve how much volume there is in the solution
Applying the formula, M1L1=M2L2
16.0M HCl×L1=2.00M HCl×0.800L
16.0M×L1=1.60moles HCl
L1=0.100L or 1.00×10ˆ2ml
0.800L-0.100L=0.700L→amount of water
Example 2: Concentrated HCl is 11.6 moles/L/ How would you make up 250ml of 0.500 moles/L HCl?
11.6M HCl×L1=0.500M HCl×250ml
L1=(M2×L2)/M1= (0.500 mole/L MCl×250ml)/(11.6 moles/L HCL)
= 0.011L
= 11ml 250-11ml= 239ml→amount of water
How to solve:
Preparing Solutions Sample Problem 1
Preparing Solutions Sample Problem 2
Preparing Solutions Sample Problem 3
- thus making it easier to transport and cost fairly less money
- but have to make concentrate form from any solution from an even more
concentrated solution
Example 1: You have 2.00L of 16.0M HCl, but you need 0.800L of 2.00M HCl
How do you make it?
- numbers of moles are always constant=not changing
- only difference between the two would be there is more water in the less
concentrated solution
Therefore, Equation would be= Moles solute before=Moles solute after
M1L1=M2L2
The subscript 1 denotes “before” and subscript is “after”
Solving for L1 wouls solve how much volume there is in the solution
Applying the formula, M1L1=M2L2
16.0M HCl×L1=2.00M HCl×0.800L
16.0M×L1=1.60moles HCl
L1=0.100L or 1.00×10ˆ2ml
0.800L-0.100L=0.700L→amount of water
Example 2: Concentrated HCl is 11.6 moles/L/ How would you make up 250ml of 0.500 moles/L HCl?
11.6M HCl×L1=0.500M HCl×250ml
L1=(M2×L2)/M1= (0.500 mole/L MCl×250ml)/(11.6 moles/L HCL)
= 0.011L
= 11ml 250-11ml= 239ml→amount of water
How to solve:
Preparing Solutions Sample Problem 1
Preparing Solutions Sample Problem 2
Preparing Solutions Sample Problem 3
订阅:
评论 (Atom)